How are new Trusted Research Environments (TREs) being developed by NHS Digital for England?

Brian talked about the developments inside NHS Digital, building on their place in law as England’s Safe Haven for patient data. NHS Digital want to be the place to go for safe and timely access to the most comprehensive store of nationally collected, linkable, quality assured health and care data for secondary use.
"NHS Digital wants to be the place to go – the one stop trusted health research service – for researchers to come in and link data in a safe environment."
The mission of NHS Digital is to provide approved researchers with rapid, safe and trustworthy access to essential health and care data. Enabling timely access to data which will enable research and to do this at scale is a significant challenge. Their work so far has been driven by, and focused on, outcomes associated with COVID-19 to guide national and international decision making and recommend potential interventions to reduce the severity of COVID-19 outcomes.
This initial work on COVID-19 is now being extended into other areas, including heart disease and cancer.
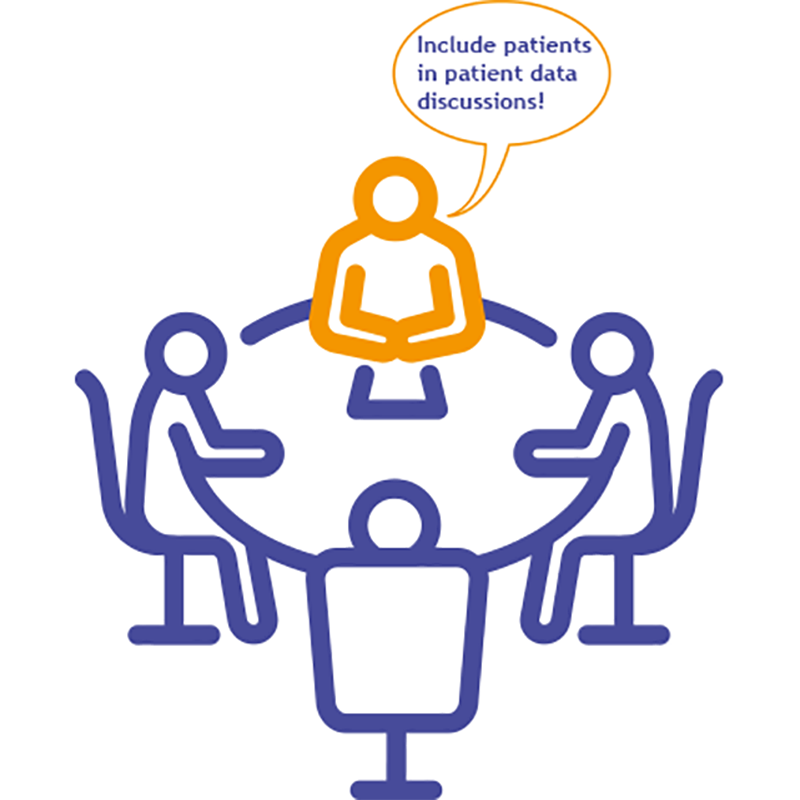
"Public and patient engagement is a watchword for the service...the PPI voice is really important to all of this."
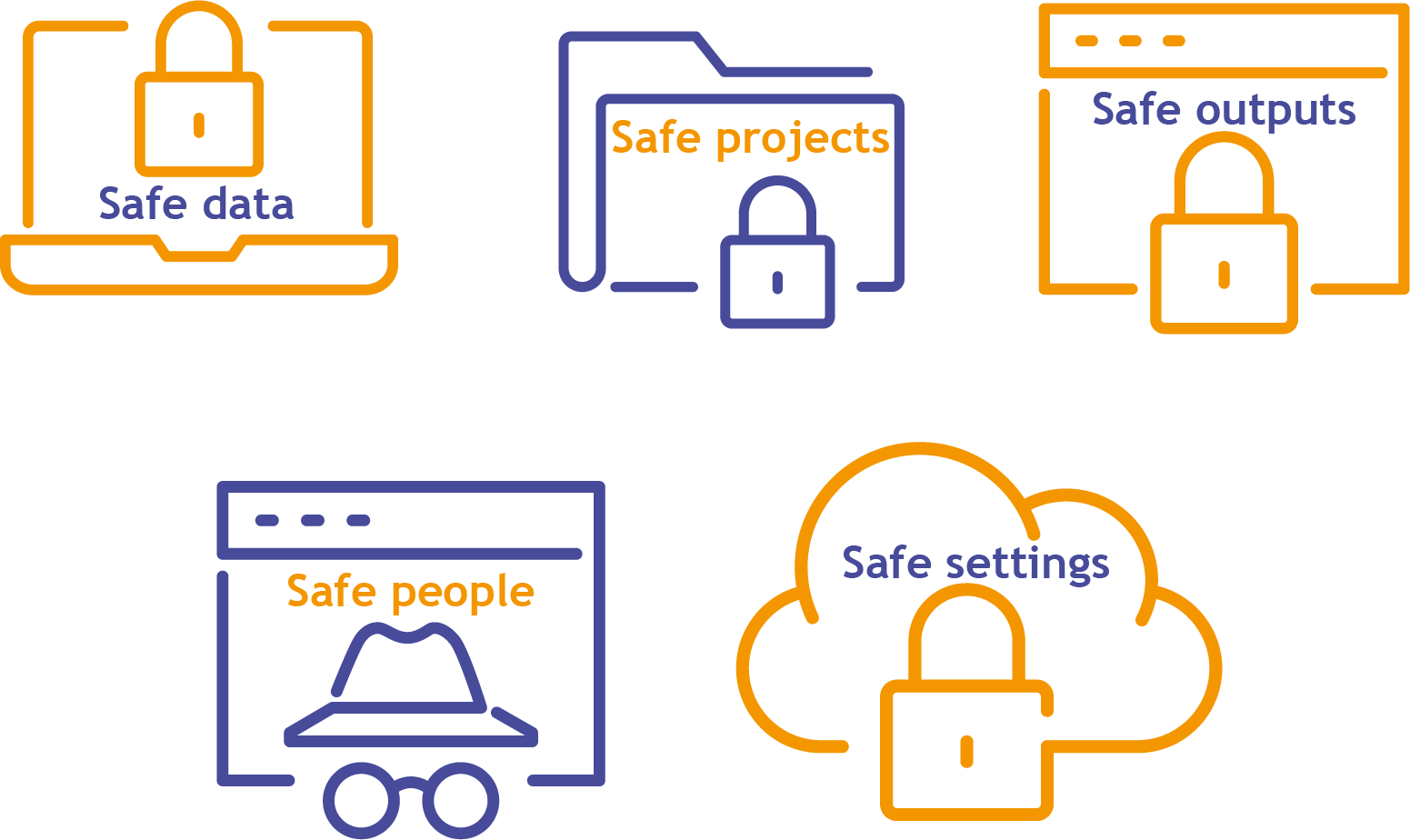
As with the other panellists, Brian described how the Five Safes were also being implemented in the design of the NHS Digital TRE solution. He highlighted the areas of development for their TRE service, which would mark a significant shift from data dissemination to (safe) access.
There remained a significant need for wider engagement. Reflecting that it was difficult to engage with everyone individually, he encouraged people to work with groups such as use MY data.
"We actively encourage people to go through groups like use MY data to have their say on things like, how we design and build the service and how we run it day-to-day"
In conclusion, Brian highlighted that their TRE development was very much at the start, and that the enduring TRE service will enable safe and timely research at scale for:
- Evidence-based decision-making
- Benchmarking (such as for a clinical intervention, a service etc.)
- Health and care surveillance/monitoring over time
- Evaluations of clinical and financial impact
- Improved care commissioning, assessment and planning.
|